Don't look now, but there are bacteriophages on your shoes. And if you scraped off a sample and sent them to a phage biology lab, one strain of them might turn out to be a natural, targeted predator for an antibiotic-resistant bacterial infection that a surgery patient has been fighting for the past year.
I'm joined this week by Jessica Sacher, a Stanford phage biologist, cofounder of Phage Directory, and founding research staff at Phage Australia. Jessica has spent more than a decade studying natural viruses that kill bacteria, trying to bring century-old ‘phage’ therapy into modern medicine—with everything from basic-science research to personally hand-preparing treatments for individual patients.
We talk about why this promising therapy got written off as "commie science," what fraction of PhD-thesis research samples just happen to cure an antibiotic-resistant infection, where they sell over-the-counter phage medication that's developed like a sourdough starter, and what it'll take to design clinical trials when every treatment is personalized to a particular patient.
Quotes
"...here's our Pandora's box of mystery phages!" (12:50)
Jessica Sacher:
I was functioning as the phage tester for doctors—
Ross:
—for the country of Australia?
Jessica Sacher:
Yeah. When we started, we had 50 Pseudomonas phages, 50 E. coli phages, and two Staph. aureus phages, and then a smattering of others. That’s not a huge library, but that’s roughly what we had...
Ross:
And of those 50, on a good day, how many hits would you get? Most of them misses, some successes?
Jessica Sacher:
We’d get something like five hits out of 50.
We had no idea what to expect, though, because we found these phages in the fridge. They were not in a nice bank ready for testing; they were each someone’s PhD project from 10 years ago or whatever. We just went into this academic lab’s fridge.
We didn’t even know if some were redundant, because we didn’t have time to do a first-step characterization. We were like, “Here’s our Pandora’s box of mystery Pseudomonas phages!”
"Oh my God, I’m not telling my PhD supervisor..." (30:20)
Jessica Sacher:
At that point, there was a University of Pittsburgh doctor looking for phages for this patient Mallory Smith. She had had a lung transplant, cystic fibrosis—it was really bad—and then got a Burkholderia infection. They wanted to kill it with phages, since they couldn’t kill it with antibiotics, but they couldn’t find phages—
Ross:
—so they post on Phage Directory, “Looking for…”
Jessica Sacher:
Well, they would have, if we’d had it by then, but we started it because of this case, launched the directory, and two days later she died. That galvanized us and everyone on the list already. They were like, “…what?”
Journalists from STAT News wanted to talk to us, saying, “Now we have something that can help…” There was Mallory’s face, her whole story—and then “…luckily there’s Phage Directory” at the bottom. We were like, “What?”, Jan said, “I’m quitting my startup!”, I was like, “Oh my God, I’m not telling my PhD supervisor; I’m supposed to be writing my thesis.”
"...less infrastructure than you'd think." (53:57)
Jessica Sacher:
In the Republic of Georgia, for instance, they’ve done phage therapy for 90 years with these cocktails that are like sourdough starters. Every year they add new phages from the environment or from clinical isolates. Who knows how many there are—maybe a hundred?
And the cocktail isn’t for Pseudomonas, it’s for “lung infection”. Or, as a patient, you get “IntestiPhage” when you have gut issues; you take it over the counter in Russia and i think some OTC in Georgia, but there’s also a clinical version prescribed by hospitals. I don’t know if the strength changes or what, I haven't been yet!...
Ross:
I think it’s fascinating, because with some therapies that are pushing the frontiers of medicine, often we need the most sophisticated medical centers—like, if you need gene therapy, I hope you’re in the city of Boston, or maybe New York, with the most specialized doctors in the world pushing the cutting edge of research. Those are who will do million dollar gene therapies.
But phage therapy doesn’t necessarily need that, if these treatments are being delivered in the Republic of Georgia—or Australia—and so the local medical system just needs to be on par with that. If you can deliver 1980s-era care, you can deliver these. There’s no extra barrier for this new therapy.
Jessica Sacher:
Yeah, that’s a really good point. People think about medical treatments as being on a spectrum from “totally personalized to the max”, versus, like, a pill. But there are personalized-medicine things that need less infrastructure than you’d think.
"...a question of trial design..." (57:30)
Jessica Sacher:
I think it still remains to be seen whether cocktails will need a diagnostic as well—I don’t know which way that will go.
Ross:
In that case, you’d diagnose by checking that the culture was sensitive to the cocktail?
Jessica Sacher:
Right.
Ross:
So that’s a question of trial design, I think. If you’re telling me that 85% of the patients—of those that reach the last line of treatment—are going to have bacteria that are susceptible to one of the strains of this one-size-fits cocktail, then we have a choice:
- We can say that we’re going to collect samples from the patient, culture them, take it to the phage lab, see if the cocktail makes spots appear in the Petri dish, and then go on to the next step in the 85% of patients that do—and for those, it will be 70% effective.
- Or we can admit patients just based on reaching the last line of therapy, not wait for the extra week-long back-and-forth to do the culture—which risks losing the patient to follow-up, and limits us to doing the trial in a hospital with an attached phage lab… Instead, we could treat all of the patients that reach this last line of therapy, and they’ll get better 70% of the time, 85% of the time—meaning that 60% of our patients are going to get better. That’s worse than 70%, but we’ve cut this whole operational step—and potential point of failure—out of our trial design.
Jessica Sacher:
Maybe that’s because you see a susceptibility-testing step in some of these trials and not others—I can see the argument for saying, “Nope, I don’t want to know!” and only checking retrospectively...
“Oh, can I do the hands-on part?” (1:08:57)
Jessica Sacher:
[Stanford] is a much more academic environment, with undergrad students just milling around. You can't imagine checking your endotoxin count and seeing next to nothing, but eager and excited students are knocking over your media and asking, “Oh, can I do the hands-on part?”...
...and don’t get me wrong; I love the students, they’re so excited! But like they’ll just pick up a vial of phage and say, “This says T4. I think I can use it because my project’s about T4!” I’m going to have to start labeling things differently just to hide them. There’s a point where it’s not compatible with academia to have an operational mini pharmaceutical unit for phage therapy.
Timestamps
1:36 - Introduction
2:14 - What are phages?
3:28 - What is phage therapy?
4:34 - Phages vs antibiotics: A history
7:31 - The limits of antibiotics
12:25 - Step 1: Test for effective phages
15:45 - Step 2: Purify the phage sample
21:12 - Step 3: Get per-patient approval
24:13 - Step 4: Treat the patient
28:13 - Founding phage.directory
33:09 - Toward broader adoption of phages
46:13 - What will commercial development (have to) look like?
53:57 - Where are phages used today?
57:30 - What if you treat, then test?
1:01:11 - Why (else) do phage therapy trials keep failing?
1:05:32 - Future directions
Transcript
Introduction (1:36)
Ross:
Welcome to Development & Research, where we talk about doing things differently in the world of clinical trials. I'm here with Jessica Sacher—a phage therapy biologist—for whom my first question is: “...what?”
Jessica Sacher:
(laughs) I've never heard it described like that, but I like it. Well, I started as a biologist who realized that the “phages” I was studying could be used in people, and then started going down the road of figuring out how to do that. And that's why I'm a phage therapy biologist. I'm gonna own that.
What are phages? (2:14)
Ross:
So let's rewind one and a half steps. It’s a scary sounding word—so what are “phages”?
Jessica Sacher:
Phages are viruses. They are scary sounding for that reason. But they’re the ones that kill bacteria, so they’re not the kinds that kill human cells or animal cells.
Bacteriophage is the full name—bacteria eater—and they look like little lunar landers. They have a head, a tail, and spider legs. And they’re pretty huge compared to human viruses. They’re just massive.
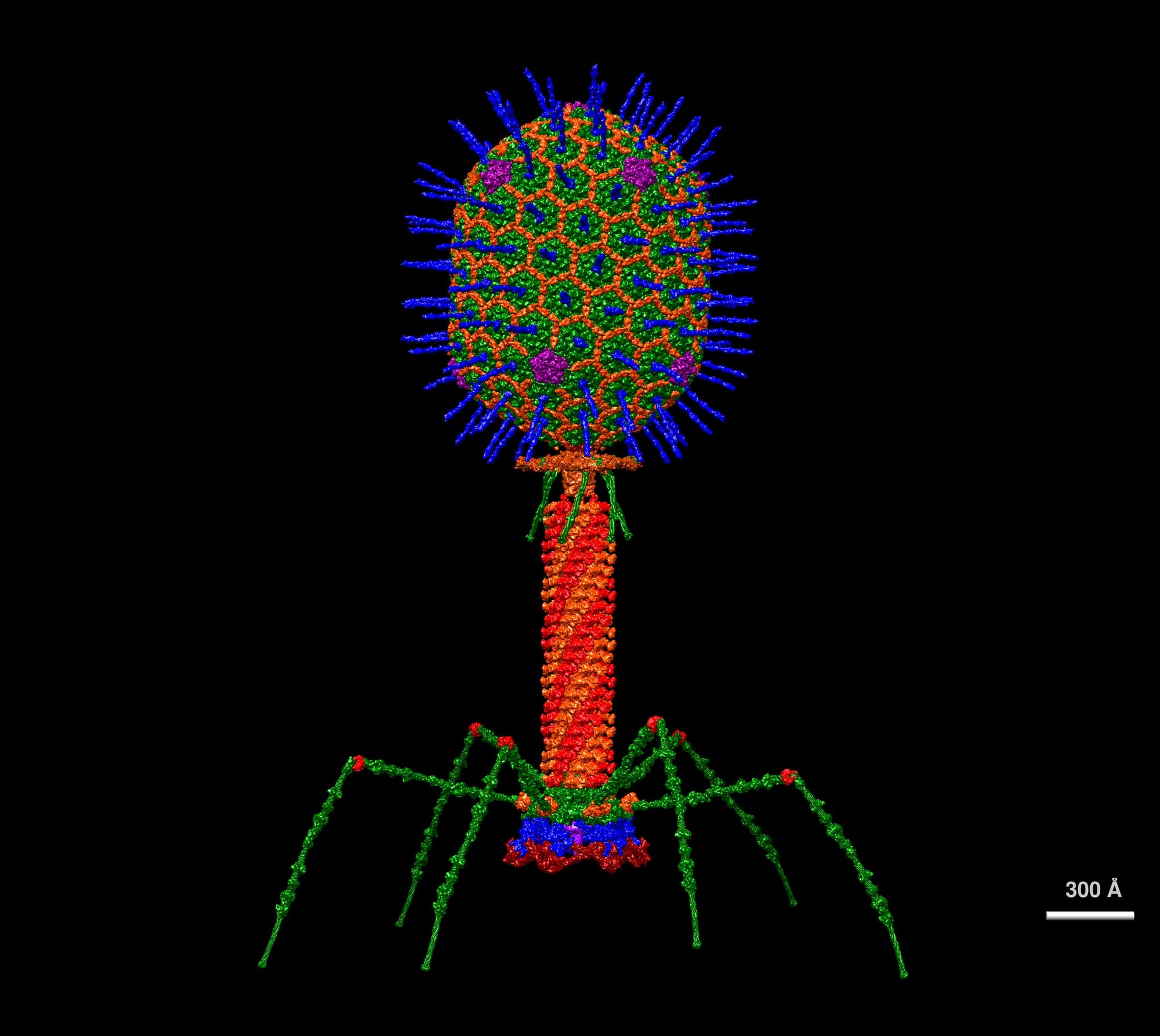
Ross:
And phages are…rare on the microscopic scale? Everywhere around us?
Are the bacteriophages in the room with us now?
Jessica Sacher:
Yes, they are. They’re mostly in our bodies, but they’re definitely everywhere because everywhere people sample, it seems you can find them—
Ross:
—the same way we find bacteria everywhere.
Jessica Sacher:
Exactly.
Ross:
There’s this entire ecosystem of bacteria around, and that ecosystem includes bacteriophages.
Jessica Sacher:
Yep. Anywhere there’s any kind of moisture and any kind of food, which is—on the microscopic level—just everywhere. Maybe not so many on the floor; like, maybe if I swab this floor, I wouldn’t be surprised if I found nothing. But on our feet, our shoes, I bet there’s probably stuff in those plants, the soil. Anywhere there’s soil, water, and any kind of microbiome, they’ll be living amongst their bacterial hosts.
What is phage therapy? (3:28)
Ross:
So when we’re talking about phage therapy then… what is it trying to treat? How is it making humans better? And how is it different from just being exposed to the phages in the world around us?
Jessica Sacher:
Phage therapy means, well, phages are being used therapeutically.
It’s kind of intuitive, and this is why it was discovered in 1915. Basically, as soon as it was figured out that there were bacteria growing in a Petri dish and there were holes in them, it seemed like, there’s something there. We didn’t have the microscopes to see it, we didn’t have the genome sequencing to know it was a DNA-containing virus, but we could see that something was killing bacteria.
So that something was put in rivers and given to people to save kids from diarrhea—people surmised that if there’s bacteria that are causing disease, and this is killing the bacteria, maybe giving this would treat that. And it did…sometimes. A hundred years later, it’s still kind of the same process: the idea of purifying a bit of the right kind of virus, and then anytime you want to kill a certain kind of bacteria, you can use it like a drug.
Phages vs antibiotics: A history (4:34)
Ross:
So this story starts very similarly to the story of small-molecule antibiotics where it's like, “Hey, we found holes in our Petri dishes.” It turns out, sometimes that’s from fungi that are making weirdly-shaped molecules. We didn't understand that at the time, either—it's just, here’s some stuff killing bacteria; if we grind it up, people take it, they get better.
We’ve since learned that it’s a molecule that binds to a protein and stops something from happening [in the lifecycle of the bacterium]—so too with phages, where we started investigating something happening in nature that was killing bacteria, through developing that as a therapy today.
[Ross: More about what makes a molecule a drug from earlier in the season.]
But that’s where these stories start to come apart, because the small-molecule path became the default for pharmaceutical development. Sometimes we have to remember there are things besides small molecules—and sometimes we need to remember there is even such a thing as phage therapy. So where in history did these stories diverge?
Jessica Sacher:
There are so many people who have written about the history, and it’s become a trope—every phage researcher, in the first paragraph of every paper, does the same paragraph. It’s like a rite of passage in the field.
Ross:
Okay, so give us the paragraph!
Jessica Sacher:
(recites) 100 years ago, phages were discovered, and then they were abandoned when antibiotics were discovered, because antibiotics work so much better. They’re much more broad-spectrum, so you don't have to be right about which one—it’s much harder to be wrong. Antibiotics will just kill most bacteria.
Especially during the World Wars, there were all these infections on the battlefield, and there's much more need to have something [general-purpose] to combat them. Antibiotics were much more scalable, and were also discovered around those same decades.
Jessica Sacher:
“But phage therapy kept going behind the Iron Curtain…”—back to that classic paragraph of phage history—Stalin was really into them, and there was a lot of use of phages [in the Soviet Union]. The West found antibiotics and used them, but nothing changed for the Soviet side, so they kept using phages just like they had, and so soldiers would have little vials on their belts to pour them on battlefield wounds—things like that.
You have to dance around the historical side of it today, because there’s this idea that it was “commie science”, and so there’s some bias against it. That’s one reason the fields stayed diverged—it took a while for the West to exhaust the utility of antibiotics and say, “Wait, we had something very targeted, and now we need something targeted when these antibiotics fail.” Meanwhile, places like Russia and Georgia had been using them in the clinic the whole time.
So roughly that's the history.
The limits of antibiotics (7:31)
Ross:
So, moving from a focus on battlefield infections—no one’s testing anyone, no one’s seeing the inside of a clinic—to today… In the developed world, people fighting serious, acute infections are going to see a specialist, who’s going to take some samples, culture them, and figure out what works for you as part of an actual clinical treatment, not just something you keep on your belt for when you get cuts.
Jessica Sacher:
Personalized medicine is a thing now!
Ross:
Right! Personalized medicine is, increasingly, a buzzy thing.
So, how does that translate into what the landscape looks like for phage therapy development today? What is driving the interest in it and what are its challenges relative to antibiotics, which you say we’re reaching the limits of?
Jessica Sacher:
First, there are starting to be more antibiotic-resistant bacteria in hospitals—and in patients.
Infectious disease specialists will have a list of antibiotics to use for bacterial infection—first the penicillins of this type, then the cephalosporins, and so forth. It’s maybe 20 classes. They go through first-line, second-line, third-line…
Jessica Sacher:
That list, by the way, changes from hospital to hospital, region to region. Someone decides, “Okay, this is the up-to-date list that Red Deer County Hospital is going by: use this antibiotic if you have this, and this other one if the first one doesn’t work…”
They go through that roster, and the drugs are getting more toxic as you go down, because you want the safest, most effective ones first. Then there’s stuff like colistin, which causes kidney disease, and you really don’t want to use that unless nothing else is working.
So infectious disease physicians around the world at major hospitals—like the University of Pittsburgh transplant unit—who have access to all the resources you might want, are sometimes out of options completely. Their patients have nothing left. Nothing on the roster will work, or the only things left are way too toxic, or the patient’s allergic. So that’s the space that phage therapy is filling: patients that have antibiotic-resistant bacteria.
Some patients will get confused and say, “Oh, I have antibiotic resistance, like I’m resistant,” but that’s a misnomer. Unless you have an allergy, it’s not you. It’s the [strain of] bacteria that’s in you that isn’t susceptible to the antibiotics. And that's where phages can come in.
Jessica Sacher:
The second part of your question is about personalized medicine—what that looks like with phage therapy. There, it is really about picking a treatment not because of a roster, but because of this patient and precisely what they have.
For example, a patient might have a lung infection like pneumonia. The doctor orders cultures, someone comes and gets a swab, they grow it in the lab, and they find out, oh, it's Pseudomonas aeruginosa, a common lung pathogen. Then with phage therapy, you’d need to take that exact Pseudomonas—not just the name “Pseudomonas”; you have to physically have a sample from that patient, growing on a Petri dish.
Then you have to have a phage on hand—though, hopefully you have multiple phages, because they’re so specific. One phage will target some Pseudomonas aeruginosa…but maybe not that exact Pseudomonas aeruginosa. We don’t know what their rules are, and that’s an active area of research. And so, if you had 50 Pseudomonas-targeting phages, or a thousand, or five, you’d test them each—pipette them onto the Petri dish, just like in 1915 with the holes in the bacterial culture. We literally do that assay.
Well, I use “we” loosely, because most hospitals do not have this capacity right now. But if the doctor is lucky enough to know a phage biologist, and has a stable patient who can wait long enough to mail the Petri plate—literally wrap it in wax, put it in a plastic bag, and mail it to me, a phage researcher at an academic institute—
Ross:
—so that you can pipette the phages.
Jessica Sacher:
—so I can pipette my phages onto it and see if any of them work. But it can be amazing because when they do, we just say, “Okay, we have the drug,” and it’ll probably work if we put it in the person.
Step 1: Test for effective phages (12:25)
Ross:
So can you set the scene here with the scope and scale? This doctor is lucky enough to know you. They take a culture from their patient, wrap the Petri dish with the culture, send it through the mail to you, and you're going to reach into your library of 12—1,200?—phages to start this testing process.
Jessica Sacher:
Yeah. When I was doing this for the last two years in Australia, I was functioning as the phage tester for doctors—
Ross:
—for the country of Australia?
Jessica Sacher:
Yeah. When we started, we had 50 Pseudomonas phages, 50 E. coli phages, and two Staph. aureus phages, and then a smattering of others. That’s not a huge library, but that’s roughly what we had.
Ross:
Sure. And you're going to test all 50. Your researcher is going to be like—
Jessica Sacher:
—yeah, we’d check them all.
Ross:
And of those 50, on a good day, how many hits would you get? Most of them misses, some successes?
Jessica Sacher:
We’d get something like five hits out of 50.
We had no idea what to expect, though, because we found these phages in the fridge. They were not in a nice bank ready for testing; they were each someone’s PhD project from 10 years ago or whatever. We just went into this academic lab’s fridge.
We didn’t even know if some were redundant, because we didn’t have time to do a first-step characterization. We were like, “Here’s our Pandora’s box of mystery Pseudomonas phages!”
Ross:
So you just have them in a numbering system?
Jessica Sacher:
They just had Sharpie on the top of the vial, some letter-number code. We hoped it would be in some Excel sheet somewhere, but we thought, “We’ll find that later—”
Ross:
“—when we need to write the paper”, or the case study, or whatever it is.
Jessica Sacher:
Exactly. But we’d get five hits, or sometimes two, and we often did not get zero, which was shocking. We thought we’d need a thousand unique phages to hit that patient isolate.
But for some reason—maybe luck, maybe it’s normal—that was our collection for Pseudomonas.
Ross:
And so—the majority of your Pseudomonas patients—with this library of 50 that wasn’t particularly designed, just compiled from people’s leftover research, most of those patients had some phages that created little holes on their personal Petri dish.
Then you’d send maybe five samples—separate or mixed on the other end—and for a lung disease, the physician on the other end is giving it as…an inhaled treatment?
Jessica Sacher:
Well, that’s where you’d want it to go for sure, but the one thing we didn’t have yet was—we literally had one milliliter of each. And that milliliter is probably not pure. It could be a mixture of phages. We ended up finding that sometimes it was a mixture, so we later had to divide them up.
Step 2: Purify the phage sample (15:45)
Jessica Sacher:
But, more importantly, they also weren’t purified of bacterial debris, so that’s going to be the next step of the process.
Ross:
Because they were grown in bacteria, then exploded out of the bacteria, but we still have all those bacterial parts sitting in our milliliter of solution.
Jessica Sacher:
Basically, yes. We call that a lysate. (Jessica: It lysed, so it’s a lysate.) We’d sterile-filter it, with holes small enough so that living bacteria wouldn’t come through, but everything else was there. There’s chunks of bacterial cell wall, and those are very dangerous.
That’s actually the most dangerous part of phage therapy—elements of the cell wall called endotoxin, that are part of the lipid layer on bacteria. That alone can cause anaphylaxis, because the human immune system really wants to find bacteria—it’s trying to save you from them and that’s one of its sensors is detecting endotoxin.
Ross:
So when your immune sensors find all these raw pieces of bacteria lying around, the immune system says, “Oh no, oh no no no,” and you get a lot of inflammation.
Jessica Sacher:
Exactly; it literally could kill them! So that was what kept me up at night: getting rid of endotoxin so we wouldn’t kill somebody.
[Ross: Endotoxin in bacterial lysate also made an appearance in our episode with Brian Finrow, when we discussed the importance of separating it from insulin grown in E. coli.]
Ross:
So I know only a little about phage therapy, but I was told that a major problem with delivering a bacteriophage is if it kills all the bacteria in someone at once, and then we have this immune reaction to all the immediately lysed bacteria.
Is that the right or wrong picture? Should we be more worried about the lysate that’s coming in the injection, or what’s produced in the patient?
Jessica Sacher:
That’s a really good point, and I think both need to be considered. But to start with, it’s present in the lysate, and we have to clean it up. You can do that with a series of purification steps and filters; we’ll talk about that.
Ross:
Though, some of the earliest trials failed to do that—and that’s where we get some of our scariest cautionary tales from.
Jessica Sacher:
Yes! People just started doing this! (nervous laughter) We need to talk about that.
But there’s also the release of endotoxin from the target bacteria—which is what you want to happen, because the target is exploding. You give the phage, it finds its target, injects its DNA, and explodes [the bacterium].
Ross:
We explode all the bacteria in you all at once—sounds great!
Or, maybe…you’d prefer it not be all at once, if your body needs time to process the endotoxin and the other bacterial parts being released.
Jessica Sacher:
Though I feel like that is not as big of a problem as you might expect. It’s a valid thing to be worried about—and I am worried about it—but we just don’t see people having many negative reactions to phages, even when they’re given as unclean preps, or when they presumably work because the patient got better.
If a test shows that the patient has less Pseudomonas, presumably the bacteria died, but we’re not really seeing—phages have been resoundingly safe, as far as medical treatments go. If a patient has a reaction, it [usually] passes soon, and they don’t die.
There are no cases of patients dying from phage therapy, and so sometimes I wonder about, are we too worried about adverse reactions if they’re not happening in the clinic? The numbers might not be reported, but—still, the concerns are valid.
Ross:
So you’re doing the prep. You have this research sample which is just this lysate, and you have to get the endotoxins out because you don’t want to be injecting them in people. But I’ve read methods sections in some of these papers, and oh boy, the methods section—you prepare the samples, centrifuge this, put it through that reduction column, this dilution, and so on…


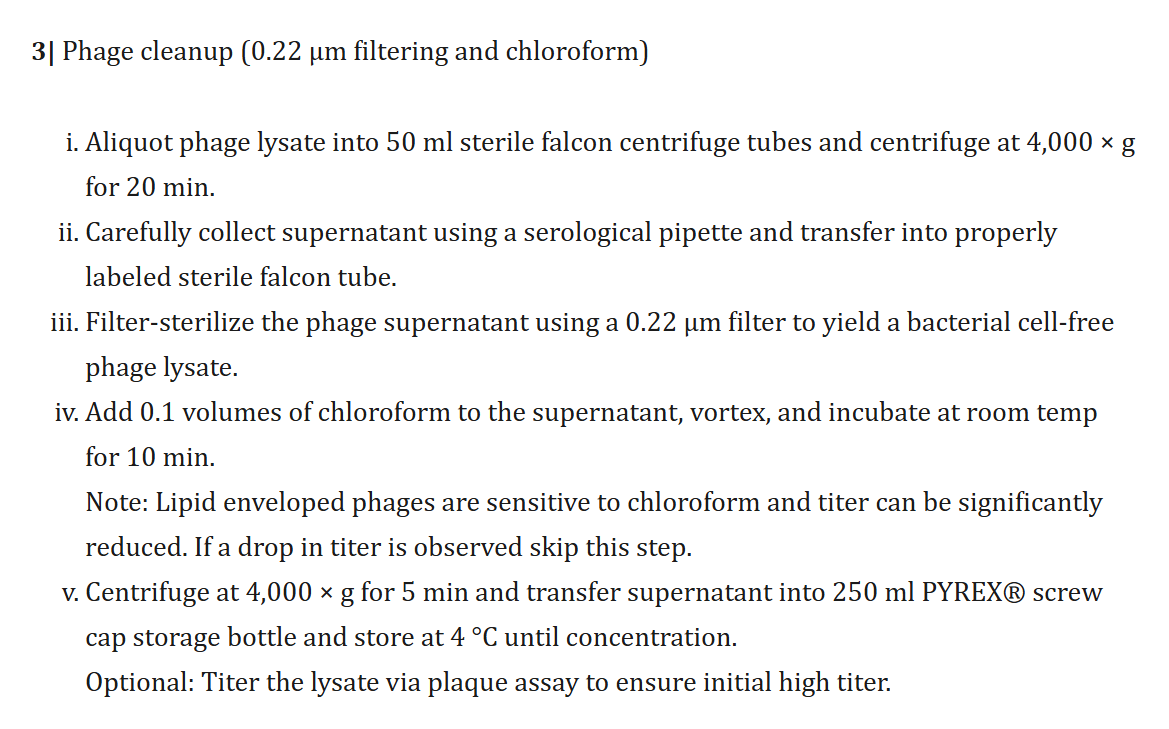




Bonilla et al. (2016), CC-BY 4.0.
Ross:
Those are the steps you’re doing at the Phage Australia for just one patient?
Jessica Sacher:
Yeah, we had to figure out how to do all that.
If you’re a company making a drug, you invest in your system for producing the phage under Good Manufacturing Practices. Maybe you have a bioreactor, because you’re only making one thing in large batches. But we were doing it case-by-case, and that was going to be the case for a while. It wasn’t always just a one-off, but we were in an academic lab with no real budget to buy machines. So coming up with an “academic version” of the process but still ending with a clean product was task number one.
Luckily, since it’s such an old field, the literature is full of methods. There are cheap ones like octanol, an organic solvent that you just mix one-to-one with the lysate; it separates, you take the aqueous layer, and most of the endotoxins are out. Then you can dialyze that, and that’s usually good enough, actually.
Step 3: Get per-patient approval (21:12)
Ross:
So the methods are very established—developed over a century in former Soviet parts of the world—and so now we have a decidedly non-GMP process: you were pipetting this sample in your sterile hood, and then we’re giving this to patients? Is that okay?
Jessica Sacher:
It’s the realm of compassionate use (or other versions of that name). We had a channel like that in Australia. You only qualify for phage if you’ve tried standard of care and it failed, and your doctor says—
Ross:
“The standard of care” means, the whole checklist?
Jessica Sacher:
Yes, and the doctor really has to put their medical license on the line. They have to say, “I’ve tried everything.”
And sometimes the standard of care is surgery—like, for a prosthetic joint infection, the standard is to scrape out the biofilm, dump in antibiotics, and sew it back up…a couple of times.
But whatever standard of care is, they’ve done it, and if you still have a chronic or recurrent, life-threatening infection—that’s the only way to qualify for that path. At that point, doctors can reach for non-approved treatments if there’s nothing else.
Ross:
Though this is very much physician discretion—physicians sticking their neck out for patients.
Jessica Sacher:
Definitely. And their hospital—their employer—is on the hook too.
Ross:
They’re taking the risk, sticking out their pocketbook for the patient.
Jessica Sacher:
Yeah; often the doctor might want to, but has to convince whoever at the hospital committee that covers risk management and insurance.
Ross:
Okay, so our patient has gone through many stages. They’ve got a doctor who’s familiar with this field—who hasn’t written it off because of its connections with communism—and who’s connected with this network of academic centers that do phage testing and prep. They’ve gone through their whole standard of care, their hospital’s experimental treatment risk management committees…and they’ve come to you for phage testing.
That testing is the easy part, I guess! You get these hits through a bunch of work, but it’s work with established procedures. Though I hope you didn’t do all of it yourself…you said you were training your researchers?
Jessica Sacher:
There were two of us.
Ross:
…oh boy.
Jessica Sacher:
We did one patient a month for the first year, and that’s all we did.
But the next iteration of that was, they brought in a few more people, and now it’s becoming slightly more of a machine—though it’s still very manual.
Step 4: Treat the patient (24:13)
Ross:
So it’s not exactly in the context of a randomized controlled trial, but—what’s the [success rate] for these patients who have gone down the whole checklist, including extreme measures, and the doctors put their licenses on the line? What happens to the patients?
Jessica Sacher:
This is really cool, because—there’s a pretty high rate that something good happens to the patient at that point. If you can get the phage, if you can do everything else—you’re more likely to fail on those steps than on the phage itself.
We find that, once you do get an in vitro hit, and that phage goes back into the patient, the patient usually does better clinically and/or better microbiologically—sometimes eradicating the infection to the point that it cultures negative. That’s awesome where previously, they were dying of that infection and not responding to anything, it usually works—after everything else has failed.
The number that keeps coming up for case studies is around 70%—when people compiled 100 case studies across multiple centers, something like 70% of patients had clinical improvement, and 60-some percent had microbiological improvement or eradication. That doesn’t prove it’s 70% effective, but that’s the number of cases—
Ross:
—these cases are open-label. We can worry about placebo effects, and—
Jessica Sacher:
—exactly. So I was wondering—everyone in the field is wondering—how many negative cases are out there, not reported? We all know you’re not likely to write a publication unless you really feel like it’s important to publish the negative cases—we should all strive to do that, but it doesn’t usually go to the top of the priority list. So I always wondered, is it drastically overreported on the positive side?
Then I was part of 14 cases in a row, and 12 were pretty good or great, two were neutral, and none were bad. Then I thought, “Okay, I would have seen if we were hiding negatives.” But I didn’t fully convince myself until then—until the first patient that I made the phage for, and then saw the results. That’s when I finally thought, “I think phage therapy can work.”
I’d always wondered—there are a bunch of cynical people who tried to build a company and failed, people who are still doing companies, and you think, “It seems promising but hasn’t panned out for 100 years? Yeah, maybe it’s not that great.”
So I guess I needed hands-on proof that it was working. I mean, it could’ve been a fluke, but—
Ross:
—14 patients is something!
Jessica Sacher:
Yes. I feel like it wouldn’t be talked about at all if we didn’t have a high record of good outcomes.
Ross:
It’s facing such headwinds that—
Jessica Sacher:
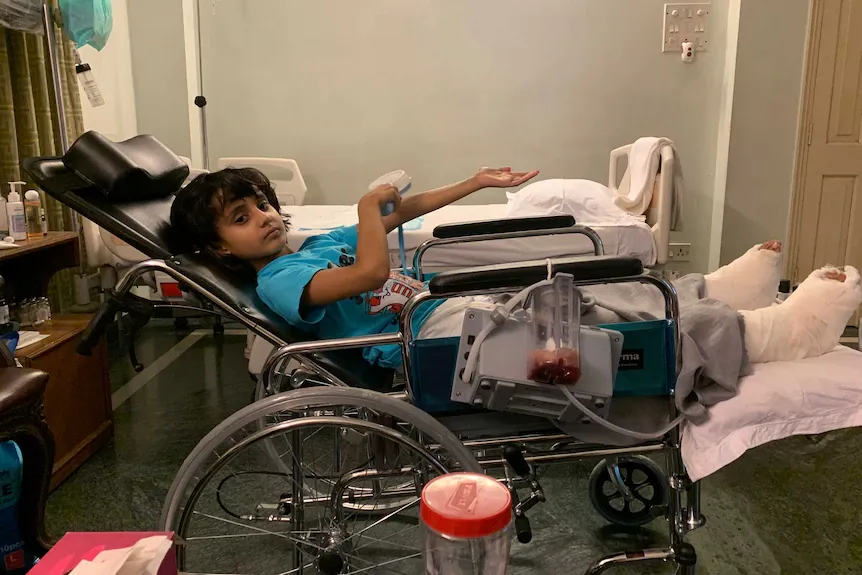
—yeah. But it keeps showing—we saved this girl who was going to have her leg amputated, and they were definitely going to amputate it, and we definitely got it so that she didn’t have an amputation. Or there was this man who was shopping for a wheelchair because his leg was going to get amputated at the Mayo clinic—it’s not just a one-off story. You can get rid of some bad infections, as long as you have a hit with a phage in a test tube.
There are honestly more headwinds in a patient getting the opportunity to have their bacterial culture tested at all—that’s the hardest part.
Founding phage.directory (28:13)
Ross:
I want to eventually talk about the gap from you two, mixing one culture at a time for patients who find you, to a world where my doctor—who may not be particularly heroic—treats me with this if I need it. There's a big gap there.
But first, let’s rewind to what it took to reach this point where you are in the lab doing these preps, convincing a doctor to take a chance. What was it like going from zero to one?
Jessica Sacher:
Australia wasn’t the first—but it was the first time that I was physically being the chemist in the lab. When I started Phage Directory in the last semester of my PhD, 2017, my partner Jan [Zheng] convinced me to help people get phage therapy on the internet—

Ross:
So it’s “Uber for phage therapy”?
Jessica Sacher:
Kind of. (laughs) He’d been in startups as a software guy, and the startups would fail or be boring or help people get food faster, whatever, and he was just like “ugh”. Then he found out that my field needed a lifesaving thing that was locked in academic labs, labs that weren’t talking to doctors, and doctors not knowing about the labs—and he said, “That’s an actual, worthwhile use of my website-making software skills.”
Ross:
So I guess it’s not “Uber for phage therapy”…more like “Craigslist for phage therapy”—
Jessica Sacher:
Yes, because we didn’t even have that!
People used Twitter, actually, or—one person used Twitter, we happened to see it, and said, “Oh. We have that!”
This is what hope looks like everybody! 211 re-tweets and several labs agreeing to help Mallory and her parents! I LOVE TWITTER!! thanks to all of you who have reached out. We will keep you all posted on the outcome of the crowd-powered phage hunt. pic.twitter.com/AguIoBaHHK
— Steffanie Strathdee, PhD 🗡️ 🦠Superbug Slayer🗡️ (@chngin_the_wrld) November 9, 2017
Ross:
Lucky that you were there, following that.
Jessica Sacher:
Completely lucky. And Jan was like, “Oh, why don’t we just put everyone that you know who has a lab on a list? Then try to get doctors on another list? And if they want to ask a phage, we just email everyone.”
Ross:
Sounds pretty good!
Jessica Sacher:
Yes, so that was the plan. And we did it.
At that point, there was a University of Pittsburgh doctor looking for phages for this patient Mallory Smith. She had had a lung transplant, cystic fibrosis—it was really bad—and then got a Burkholderia infection. They wanted to kill it with phages, since they couldn’t kill it with antibiotics, but they couldn’t find phages—
Ross:
—so they post on Phage Directory, “Looking for…”
Jessica Sacher:
Well, they would have, if we’d had it by then, but we started it because of this case, launched the directory, and two days later she died. That galvanized us and everyone on the list already. They were like, “…what?”
Journalists from STAT News wanted to talk to us, saying, “Now we have something that can help…” There was Mallory’s face, her whole story—and then “…luckily there’s Phage Directory” at the bottom. We were like, “What?”, Jan said, “I’m quitting my startup!”, I was like, “Oh my God, I’m not telling my PhD supervisor; I’m supposed to be writing my thesis.”
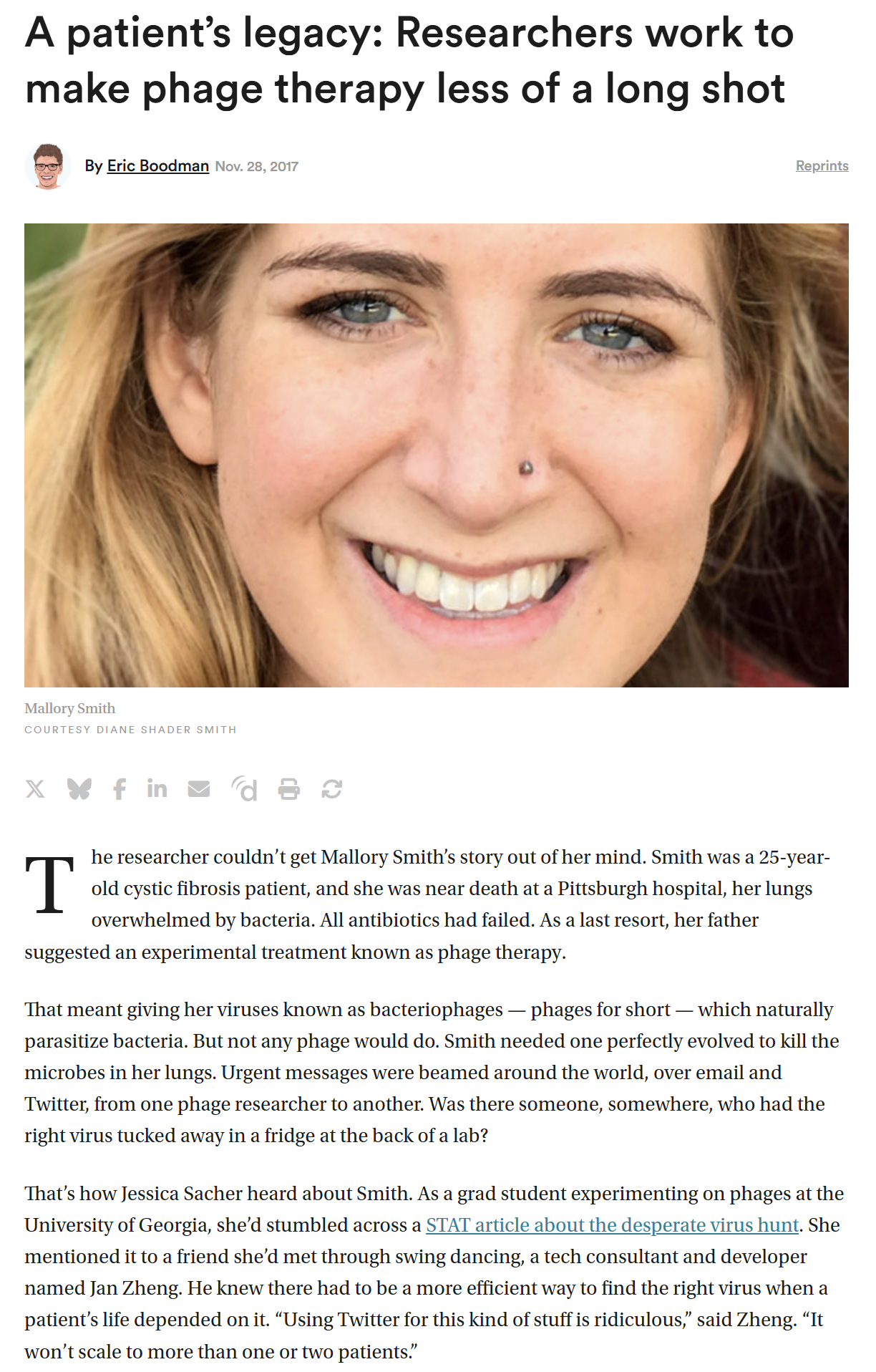
Jessica Sacher:
So that’s how bad and ridiculous the world was—even though Mallory had a doctor who was willing to look for a phage, when most doctors were not. That was also the hospital that had let her get a lung transplant when most wouldn’t, because [risk committees] will say no to that, too.
So, painting the picture, from Mallory’s case in 2017 to 2022—when I started making phages for patients in Australia—
Ross:
We went from Twitter—maybe—to PhageDirectory dot…com?
Jessica Sacher:
It’s at phage.directory. But—the world has changed a lot. It used to be that you were luckier than lucky if you were a patient who convinced their doctor to try this. Even in 2022, it was just becoming more normal for an infectious disease doctor in the US, Australia, and Europe to know about phage therapy. Europe deserves a lot of credit, by the way, with Belgium as the main driver of the field.
But patients email us all the time, wanting phages. We would tell them, “Get your doctor on board,” and they’ll say, “I can’t, he says no.” So I’d say, “Send them this PDF,” and they’d say “I will, thank you so much, no one else will even answer me!” and they’re so grateful. It continues like that, today.
And then some of these patients would come back to us two years later: “I got my doctor on board!” And it’ll be the same infection after two years, because some are very chronic—or after surgery, it keeps coming back, or [cystic fibrosis] patients are constantly being re-infected…
Patients are driving it. Doctors used to be just “No.”—then some doctors got to, “Okay, but only if asked.”
Toward broader adoption of phages (33:09)
Jessica Sacher:
Then the next shift—and Phage Australia—came about because one of these cases actually worked—
Ross:
—they made the connection, and then the hospital said, “We should actually do this”?
Jessica Sacher:
Well, it was the research lab—John Iredell is an infectious disease doctor who runs a research lab [at the Westmead Institute for Medical Research]. He said, “I want to treat more patients with phages like this,” but he first used our system completely by fluke because one of his researchers met Jan and me at a conference.
Ross:
—and he remembered “Phage Dot Directory,” found some other lab who sent him a sample that someone purified—
Jessica Sacher:
Yes, exactly. It came from Israel, went to the US to get purified, then went to Australia. It was wild. We didn’t think anyone would help us in Australia because shipping costs would be too high, but 12 labs said they would!
Anyway, we treated this girl, Dhanvi, and she didn’t have her leg cut off—

ABC News (Australia), fair use.
Ross:
—so then John Iredell thought, “Okay, let’s take this seriously—”
Jessica Sacher:
“—let’s do it.”
And it was because of his credibility as a pretty senior infectious disease specialist—he wasn’t just some new doctor saying, “I think we should use phages!” He was like, “It’s about time!” and “I need a legacy at this point!”
Ross:
The tenure system is good for something!
Jessica Sacher:
Yes! He had total credibility, which gave him a platform to do it from, and he got a big grant for making a phage therapy—well, it wasn’t called a “center”, but it had four pillars: banking phages, diagnostics, production, and monitoring.
“Monitoring” meaning—nobody had been collecting data very well, so we couldn’t do real clinical trials, and this was a pioneering move on their part, to do that. They got money from the Australian government, which was exciting for everybody in the phage field, because nobody else had gotten a million-dollar-plus grant for phage research, anywhere.
So next he reached out to us with, “Phage Directory helped us, so Jess and Jan, why don’t you come work with us? I didn’t know how…you could be a postdoc, you could be a bioinformatician…” Jan’s like, “...I’m a software developer?” They said, “That sounds like bioinformatics! Just come in and we’ll make this whole thing work.”
The big thing was not just a center, but a series of clinical trials—the first was called the STAMP Trial—“standardized treatment and monitoring for phage therapy”.
STAMP is actually what’s called a case series—each patient still has to qualify through compassionate use, meaning that they tried the entire standard of care. But they can have any [kind of bacterial infection], because the protocol didn’t specify that. They finagled it beautifully, I think, because it totally fits—even though it’s not how you’d normally do a trial.
Ross:
You mean—it’s this data collection…[framework]…which exists in parallel to the existing system for getting patients approved for individualized, experimental, last-line therapy care. But that means that all the data is being collected all in one place, together.
[Ross: Since hearing Jessica's story and looking a bit into STAMP, I've become increasingly optimistic about the case-series / process-trial model for other ultra-rare treatments that can be structured as personalized therapies within some base platform. Get in touch if you're interested in chatting more about ideas in that ballpark!]
Jessica Sacher:
Yeah. So, the (US) FDA has come to phage conferences for years—first, it’s amazing that they come to the conference at all!—and they would say, “Please talk to us, we want to work with you!” but also, “Don’t collect data from compassionate use cases in series and pretend that’s your clinical trial. Compassionate use isn’t a substitute for clinical trials in drug development.” I remember this being hammered home.
But I think other people interpreted that as “No data collection from compassionate use!” That was the sentiment that was thrown around—because [when STAMP was announced,] they were like, “Wait, you’re allowed to do that in Australia?” And I think we were allowed to do it in the US, too—it’s just that’s not a substitute, and a drug developer would still need to do a randomized trial, later in product development.
Ross:
It’s just, this is how we're going to learn how—this is how we're going to learn how to do the very first trials—
[Ross: I swear, if I had a nickel for every story I heard about FDA guidance welcoming something promising and an industry full of drug developers keeping up with the same old practices...I could buy myself a latte in San Francisco, at least.]
Jessica Sacher:
—and decide which trial to do first. Exactly.
I should also credit Ameneh Khatami, the pediatrician at the Children’s Hospital at Westmead who treated that little girl. She wrote this trial with John [Iredell], got it funded and approved—and then they got all the infectious disease doctors in Australia to sign something saying, “This is how we’re going to do phage therapy; we all agree.”
That was so cool, because no other country’s done that. I’ve been running the Phage Newsletter since starting Phage Directory, putting out five cool news bits every week for five or six years, and nobody does that. It was so cool.
So they said, “Yes, come on over,” then COVID hit right when we got funding secured. The funding got frozen, we couldn’t get visas… Eventually we did, but we amused ourselves until then by running Phage Directory, working on that, so that by the time we got to Australia, we were ready to say, “okay, we have patients!”
Because, instead of patients trying to convince their doctor, well—John Iredell is the doctor who runs every trial. He personally has 21 patients—at the hospital next door—who need this. We had all their samples, because he’d been collecting them. We just started picking things—
Ross:
You land on day one, and it’s, “Great, a 20-patient backlog!”
Jessica Sacher:
(laughs) Right. He was like, “Jess, wha’ do you think? We can give this a go?” and I said, “What do you mean?”
I actually didn’t think I was going back into the lab; I thought “I’m going to be in my role at Phage Directory, helping us get collaborators, integrating with other phage banks and get more sources of phages…” But I realized, “Oh, my phage-making skills are actually needed.”
Ross:
You had to actually pipette the titer, take the aqueous layer, and…
Jessica Sacher:
Yes, exactly.
Ross:
So there you are, you finally have your visas, and John said, “We have 21 patients, let’s—”
Jessica Sacher:
“—let’s give it a go!”
And I said, “John, what does that mean?” and he said, “Well, do you think we could just…purify the phages ourselves?”
That wasn’t decided beforehand, by the way—the STAMP trial is designed as a way to get the information from the patients, each with their own miscellaneous infections and getting their own phages. That was really cool—
Ross:
—one set of procedures; different inputs—
Jessica Sacher:
—exactly. It was the same dose by the numbers—10^9 phages per dose, the same number of doses—
Ross:
—using the same purification column—
Jessica Sacher:
—well, now that wasn’t specified. Everything with making the phages, where they come from—was not in there. The protocol just said, they will get phages that kill their bacteria [in culture] and they will get this many of them, at this interval; then we will check it by these methods…
But the question “Where do phages come from?” wasn’t spelled out, and I didn’t really ask that question either. I remember first being surprised and then thinking that I shouldn’t have been surprised…because it turned out the phages were coming from me. (laughs)
So I said, “Well, I know the literature, I know who my favorite groups are to copy if I’m making decisions about methods—”
Ross:
—which you were!
Jessica Sacher:
Yeah. And John said to give it a go.
So, I gave it a go. The first patient had an abscess in his chest where his lung used to be—it was taken out for cancer, years prior—full of E. coli. They couldn’t get rid of it, maybe because antibiotics weren’t reaching it or the bacteria were resistant.
That meant that the plan was intravenous phage therapy, so it had to be as clean as possible—
Ross:
—and so you were writing the methods for that.
So, how did you clean out those endotoxins?
Jessica Sacher:
I said, “We’ll use octanol, because I like this method called Phage On Tap by Jeremy Barr. I think he’s probably good; I’ve seen him for a few years at conferences, and I want to copy the method he published.”
Luckily it worked beautifully the first time, which tends to happen in biology—you get beginner’s luck, then it’s harder late—
Ross:
—or we just never hear about the tries that fail.
Jessica Sacher:
Yeah. (laughs)
Also, it helped that it was E. coli, because that’s the easiest organism across the board.
Ross:
It’s the easiest to culture?
Jessica Sacher:
I mean, it’s the model organism for bacteria, it grows the fastest, and it just so happens E. coli was causing this man’s problem.
Then we happened to have phages—among our 50 E. coli phages—that worked, and the octanol method worked really well to get rid of endotoxin. We found a way to test endotoxin levels very accurately, which was crucial—something I didn’t appreciate until I didn’t have that machine anymore. (laughs)
We were at the Westmead Institute for Medical Research, and they had the Sydney Cell and Gene Therapy Center downstairs. That was another fluke! But there at Westmead, they were doing CAR-T therapy in the basement, and we were doing phage therapy on the fourth floor. I just talked to people until someone pointed out that they were already testing for endotoxin, in making their treatments. Because the FDA, in the US, specifies endotoxin limits for anything injected—it doesn’t matter if it’s phage or anything else—and Australia’s TGA follows the same levels: five endotoxin units.
So I found out that there’s an endotoxin reader in the facility downstairs. And so, as long as we could prove the dangerous [endotoxin] was really low, we could argue our [purification] method was good enough. That’s what we did.
Then we treated the guy—and we cured his abscess completely. He came off antibiotics—he’d been on IV antibiotics for three years, the entire pandemic, at home—and then he didn’t need it any more. And I was like, “Oh. Maybe this stuff works!” It was wild.
So we started testing the 21 patients. Then—to your question—other doctors found out, because they know John. We weren’t advertising, but they’d say, “I’ve got a patient! It’s this guy named Tim, four hours away, in a rural area…” And once we took one Petri dish from that doctor, we got a new one every week. “This is another guy!” “Is it the same guy?” “No…I don’t think so.” (laughs) They were all Pseudomonas, but different ones—okay.
So it was doctor by doctor, but once a doctor’s into it, they’d have many patients. I inferred from that that every infectious disease doctor has multiple patients who’d benefit if they had phage therapy—they just don’t have access. It’s just laborious to get that access, and they don’t have time. But the field has shifted—it’s no longer patient-versus-doctor, it’s doctor-versus-time, and the physicians are on-board now.
What will commercial development (have to) look like? (46:13)
Ross:
Okay, so that brings us towards a better world where patients are getting off their IVs, or not having amputations. Some patients see it fail, but we have these cases, not rare, where it succeeds for people who have otherwise exhausted the medical toolbox. But there’s still a huge gulf between that and this being something that doctors prescribe within the standards of their practice, and that is covered by insurance, Medicare, or other public health systems. Conventionally, that gulf is crossed by commercial development—a for-profit company, maybe spun out of a university or nonprofit lab. That’s almost always the next step.
You described the change from 2017 to 2022 in Australia—the landscape of physician acceptance and treatment got better—but the next mountain to climb, then, is commercial development. What movement have you seen there? Are phage therapies going to be developed commercially, or will we just stay in a world where there’s a case series…if you know John…or if your doctor heard the talk at a conference? Where are we going?
Jessica Sacher:
That is the question to ask! The current struggle is, well, getting to that point [that you describe]. Since the ’90s, a lot of phage companies have started and failed. There are skeletons on the road, so to speak, go find out why they failed…
Jessica Sacher:
Look, the ideal is that you get a cocktail of phages, put them together, and now it’s a broad-spectrum phage cocktail, just like penicillin, which is a broad-spectrum antibiotic.
Ross:
You’d have doctors reach for the phage cocktail the same way they’d reach for a penicillin, not as a custom-prepared one-off. In fact, it was made with Good Manufacturing Practices, in stainless steel bioreactors, like any other drug.
So we’re going to take your 50 phages for Pseudomonas, develop them as a cocktail, and then we’re going to run (sigh) Phase I, Phase II, Phase III, Phase III again—demonstrating that this cocktail treats their last-line bacterial conditions. That’s the path to this being a by-the-book treatment?
Jessica Sacher:
(sigh) Pretty much.
Although, there are a few things that make that pretty hard—
Ross:
—of course.
So let’s get into that—what makes it not that simple, and what needs to happen differently in phage therapy than in other fields?
Jessica Sacher:
Right. The first thing that jumps out at me about your plan is, we can’t have a 50-phage cocktail.
Ross:
…why not?
Jessica Sacher:
Well, we should challenge all these assumptions! But the reasoning seems to be, someone once made one with 12, and it died off, on the shelf, quickly, while it was waiting to be used. (laughs) That’s the short answer.
That’s one example, but also consider the complexity of manufacturing. Separately from the shelf-stability—if we had 50 phages, you’d grow them in 50 different bioreactors. That seems like a pretty big barrier, and so most phage cocktails are being developed with three to six strains; that's the most that I've seen lately.
[Ross: In episode 2, I talked with Brian Finrow about the challenges of drug-cocktail development, and why commercial developers in other areas of unconventional antibiotics stop at one or two components instead of going higher.]
Ross:
The thinking is usually about finding the lowest number that might work, because each additional component is too much of a burden, especially when the path itself is unproven.
Jessica Sacher:
Exactly. So there’s this tendency towards the minimal number of phages that are going to have the broadest coverage. Fortunately, you don’t need fifty phages to get to 80% coverage—you might only need three. If we needed fifty, maybe it’s possible that we could do it—but we probably don’t have to.
So that’s one thing. A lot of people put forth that you'll still have the “long tail” of cases it doesn’t work for. In our case, we’re already dealing with the long tail of cases not treated by antibiotics, but if we make a fixed cocktail and treat the remaining patients, we might treat 80% of them, and then 20% will remain—
Ross:
—and we’ll need compassionate-use waivers for them, to try the other forty-seven phages—
Jessica Sacher:
—exactly.
And then there’s the sense of, for all the work of doing a phage product, if you’re doing it as a fixed cocktail, you’ve given up the advantage that you had over conventional antibiotics—because the bacteria can develop resistance [to the phages in your cocktail]. That causes pushback against the fixed-cocktail approach—that, at best, it’s as good as this thing that is not good enough.
I mean, fair, but I still think we can hit a lot of infections with the cocktail approach, and there should be a Pseudomonas cocktail, a Staph cocktail, an E. coli cocktail… Maybe there will be combined cocktails for the most common pathogens for a particular disease, but there are still going to have to be a bunch of different cocktails on the market, ultimately, across all diseases.
Ross:
…and across the scale of decades, we’re going to need the new Staph cocktail, the new Pseudomonas cocktail, to come through the commercial development system before the old one becomes useless.
That’s the same problem we see in antibiotic development: we’d like new antibiotics before we absolutely need them, but it's hard to incentivize that through a commercial process where everything is back-chained from decisions by insurance payors at the end—and where investors think those decisions are going to go. That can get really murky when you're trying to break new ground.
Jessica Sacher:
Yeah. Those are some of the issues, but ultimately it doesn't have to be crazily more complicated than [a new antibiotic]. It would be great if we got to the point where we had a few cocktails in the pipeline and check them for reasonable coverage of particular types of bacteria.
Where are phages used today? (53:57)
Jessica Sacher:
In the Republic of Georgia, for instance, they’ve done phage therapy for 90 years with these cocktails that are like sourdough starters. Every year they add new phages from the environment or from clinical isolates. Who knows how many there are—maybe a hundred?
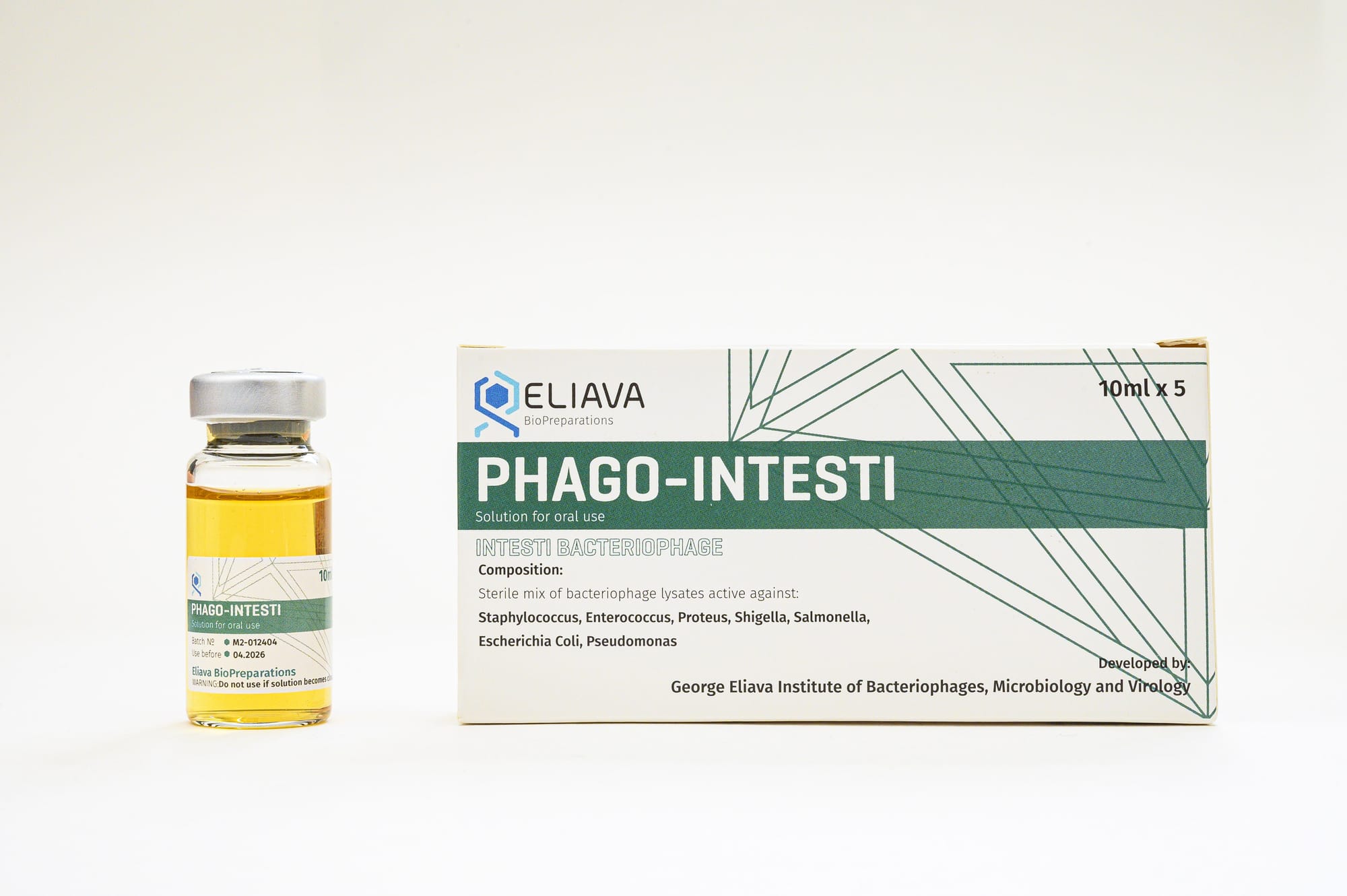
And the cocktail isn’t for Pseudomonas, it’s for “lung infection”. Or, as a patient, you get “IntestiPhage” [Ross: sometimes styled “Phago-Intesti”] when you have gut issues; you take it over the counter in Russia and i think some OTC in Georgia, but there’s also a clinical version prescribed by hospitals. I don’t know if the strength changes or what, I haven't been yet. It’s happening over there in a different way—we’re thinking of it as pathogen specific rather than disease specific. It could be simple! It’s not impossible to wrap your head around doing cocktails, and there are some companies making progress there.
Ross:
I think it’s fascinating, because with some therapies that are pushing the frontiers of medicine, often we need the most sophisticated medical centers—like, if you need gene therapy, I hope you’re in the city of Boston, or maybe New York, with the most specialized doctors in the world pushing the cutting edge of research. Those are who will do million dollar gene therapies.
But phage therapy doesn’t necessarily need that, if these treatments are being delivered in the Republic of Georgia—or Australia—and so the local medical system just needs to be on par with that. If you can deliver 1980s-era care, you can deliver these. There’s no extra barrier for this new therapy.
Jessica Sacher:
Yeah, that’s a really good point. People think about medical treatments as being on a spectrum from “totally personalized to the max”, versus, like, a pill. But there are personalized-medicine things that need less infrastructure than you’d think.
You do need a lab that can handle phage microbiology for sensitivity testing—
Ross:
—well, not if you had a cocktail, where you could make it in one lab and ship it to all the hospitals.
What if you treat, then test? (57:30)
Jessica Sacher:
You’re right, in that version of the world. Though I think it still remains to be seen whether cocktails will need a diagnostic as well—I don’t know which way that will go.
Ross:
In that case, you’d diagnose by checking that the culture was sensitive to the cocktail?
Jessica Sacher:
Right.
Ross:
So that’s a question of trial design, I think. If you’re telling me that 85% of the patients that reach the last line of treatment—are going to have bacteria that are susceptible to one of the strains of this one-size-fits cocktail, then it sounds like we have a choice:
- We can say that we’re going to collect samples from the patient, culture them, take it to the phage lab, see if the cocktail makes spots appear in the Petri dish, and then go on to the treatment step in the 85% of patients that do—and for those, it will be 70% effective.
- Or we can admit patients just based on reaching the last line of therapy, not wait for the extra week-long back-and-forth to do the culture—which risks losing the patient to follow-up, and limits us to doing the trial in a hospital with an attached phage lab… Instead, we could treat all of the patients that reach this last line of therapy, and they’ll get better 70% of the time, 85% of the time—meaning that 60% of our patients are going to get better. That’s worse than 70%, but we’ve cut this whole operational step—and potential point of failure—out of our trial design.
Jessica Sacher:
Maybe that’s why you see a susceptibility-testing step in some of these trials and not others—I can see the argument for saying, “Nope, I don’t want to know!” and only checking retrospectively—
Ross:
—because, if you decide you need to know, you can go on this entire journey to upgrade your 60% efficacy to 70% efficacy plus 15% of patients excluded without treatment—weren’t exposed to false hope, or other risks of interacting with the medical system.
There is a school of medical ethics that says that treating people with something that was futile, that’s unacceptable, but which raises the question—how much work do you need to do to make sure it’s not futile before you treat them at all? What does the Hippocratic Oath say about how much confirmatory testing you need to do before you treat your patients? [Ross: That’s rhetorical, of course—it says nothing on that topic. Incidentally, it’s also full of some kind of wild, now anachronistic, proscriptions, but that’s a topic for someone else’s podcast.]
So I can see it both ways—from the perspective of the trial designer, if they design a study that’s 60% effective instead of 70% effective, is that going to be sufficiently compelling? But the disadvantage of that 70% is that it restricts what countries you can do the trial in—whole geographies become places where you can’t do the trial—that’s a sort of theory-meets-reality tradeoff between something that comes up in the lab and the academic literature, versus something that only shows up at the very last mile, based on what buildings are linked to your hospital.
[Ross: "But my IRB sees that I do have a phage lab and says that therefore it's unethical not to do the sensitivity assay." I'm sorry to hear that, imaginary interlocutor. Have you considered running your trial in a different country that simply doesn't have access to phage labs at all? Charlie and I discussed last episode that it can be easier to get approval to trial a new cancer drug if patients' baseline option is nothing at all, rather than the standard of care at a US research institution.
If hospitals in a country simply do not have the capacity to do these slow tests, then common-sense morality and a pragmatic regulator may both agree that it's better to treat-and-bank-samples than to give patients nothing—if nothing is indeed what they would otherwise get. Then, once your trial is running in treat-then-test mode, your results demonstrate that that works, and you're halfway to making the regulatory case that it should be officially labeled for treatment in "patients with an infection" (works with p=60%) rather than "patients with an infection, after we spent several days running specific tests" (works with p=70%).]
Why (else) do phage therapy trials keep failing? (1:01:11)
Ross:
When issues cross boundaries like this, I think that both sides need to understand the other side—or else, whoever brings it forward is going to make a decision, face the consequences, and create some challenges—whether that challenge is that the efficacy is 60% instead of 70%, or the challenge is not losing the patient to follow-up while waiting for the sensitivity test, plus only getting the patients who consent to the additional procedure…
That’s the thing I find fascinating—how do these choices get made? And what choices are going to need to be made differently, [for phage therapy trials,] than the way we normally do things?
Jessica Sacher:
…now I’m re-thinking my own field!
It’s new for me to get into the clinical-trial design side, but I’ve been curious: how do these trials get designed? Because there have only been seven trials—thirteen if you’re generous—of phages—
Ross:
—most of which are safety trials; three of which were efficacy trials, two of which worked (and one of which had a shelf-stability problem)—
Jessica Sacher:
—yeah, but even those failures failed for sad reasons in terms of actually getting answers!
Ross:
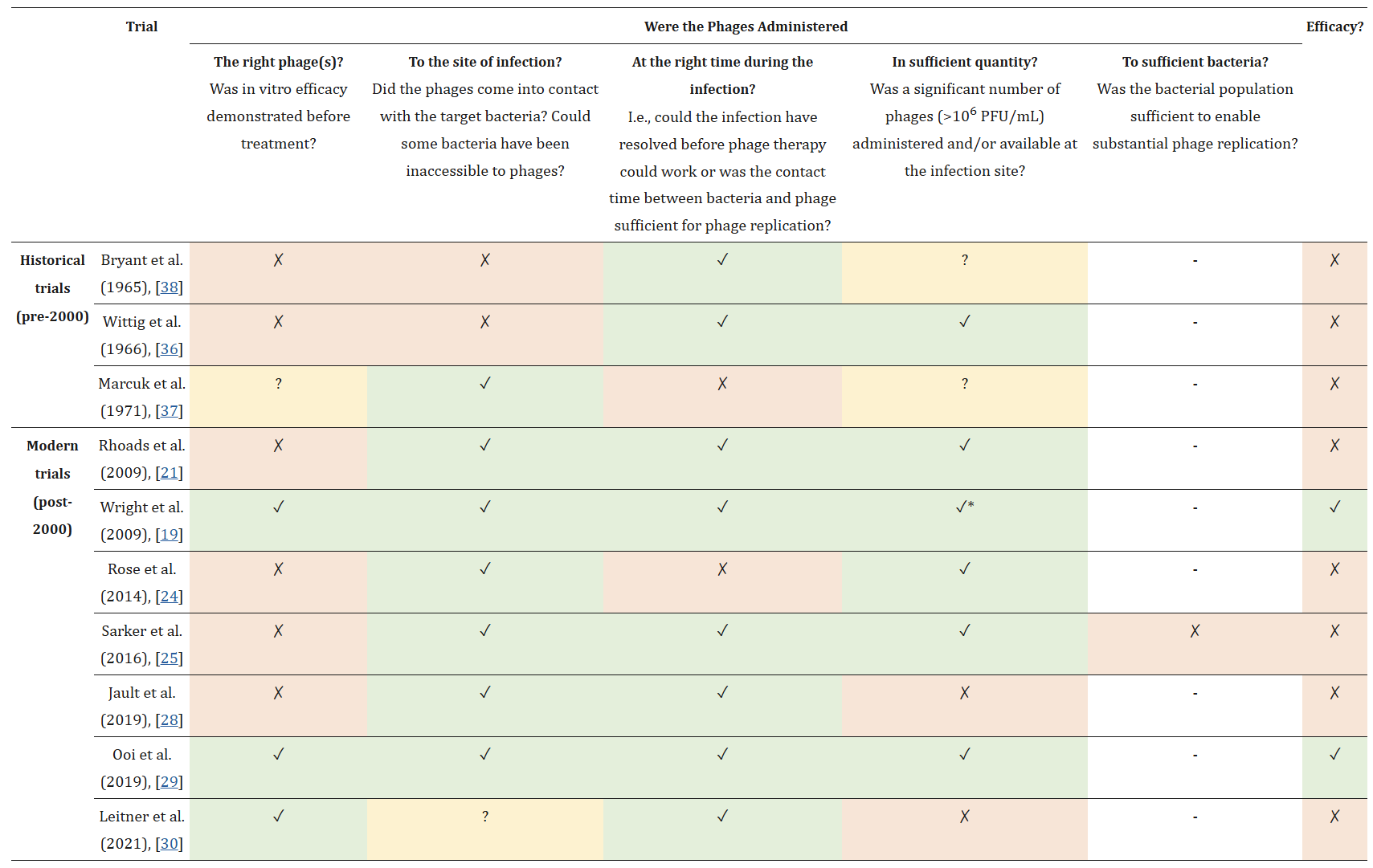
There’s a lovely chart from the paper, that’s basically “Things that can go wrong in your trial” in the columns [Ross: "the right phages" / "to the site of infection" / "at the right time" / "in sufficient quantity" / "to sufficient bacteria"...], full of—X, X, X… Two of the trials have all their boxes green, and they succeeded! But you do just have to get eight different things right in your trial, or else it’s not going to work.
Jessica Sacher:
In that paper, they called it a “constellation” of factors—and I wonder, is that how it is for everyone?
Ross:
Well, constellations are just given to us by astronomy; no one chose to put it there!
Jessica Sacher:
But my main reason for not having abandoned the phage field yet, is that I’m just not convinced that it’s not doable; it’s all just execution issues. And that’s very exciting, if it is—
Ross:
—sure! If everyone keeps failing for reasons that we know about, that’s great!
Jessica Sacher:
—and they seem like they’re fixable. I don’t mean to disparage these researchers, or claim that I wouldn’t have made the same mistake—
Ross:
—cases where the first people to try it tried the wrong thing at first, or when they did their recruitment it turned out that there just aren’t that many patients who wanted to stay for sensitivity testing—
Jessica Sacher:
—or in PhagoBurn, where they thought that the hospitals were collecting data on whether there was more than one organism in patients’ burn wounds—but they weren’t. They were just collecting [the first thing they found in the bacterial culture]—great, this patient has Pseudomonas. So the trial designers set the inclusion criteria to be that the patient only has Pseudomonas. And when they got to the trial, the physicians said “oh, there’s probably other stuff in there”, and then they couldn’t use those people. So they didn’t get enough people, it took them so long to recruit patients that their drug went off while they were waiting. Ugh!
Or the trial in Bangladesh with oral phage therapy [for gut infection] sponsored by Nestle—the phages were made against the pathogen that they thought was causing the diarrhea, and now they’re…pretty sure…that it wasn’t that one causing it after all. Cool. Hm.
Ross:
I guess that’s the other side of treating with fewer diagnostic steps.
Jessica Sacher:
I just want to find out for myself: what is the reason that these things haven’t made it? Why don’t we have a phage cocktail on the market? We should be able to get that done!
I might not know enough about the realities of clinical development, but the more that I talk to people, the more that they’re not giving me a satisfying answer that says phage therapy is doomed. So then…I can’t stop working on it!
Future directions (1:05:32)
Ross:
So, with that, what’s next for you?
Jessica Sacher:
Oh man, I don’t know.
I’m no longer in Australia. It was really fun; I miss it, but I didn’t want to live far from my family forever, so I had to quit while I was ahead and make the next transition.
[Phage Australia] is hiring a bunch more people. They’re continuing to treat patients; I think they’ve treated 40 by now? We did 12 or 14 before I left, but now they’re just cruising along. At some point they’ll try to spin a company out once they have a phage cocktail that looks right, and they’ll do a trial for cystic fibrosis or some other condition to commercialize it—at least that was the plan. There are some other companies in the US and in Europe doing the same.
But for me, I was thinking, “How can I get this kind of job on my home continent?” I looked around. I could join a phage company, but I’d probably just be at the bench doing pipetting. They have that covered.
But also—if the company makes this cocktail or that cocktail—who’s doing all the long-tail personalized cases that will need to be treated too? I see a world of phage cocktails and personalized phage therapy coexisting, forever—and there’s a lot of budding interest in personalized phage therapy centers everywhere. We’re seeing these things pop up in the US, in Asia, in South America, even in Canada. People want to replicate what we did in Australia, and I’m excited by that.
I joined Paul Bollyky’s lab at Stanford. I basically emailed him and said, “Want to do something in the phage therapy space?” and he answered, “Yes, I want to create a phage therapy center!” So I thought, Stanford’s a good place for that—you see their things all the time in the news—and I got the sense that that would be good.
Well, they have resources, support structures. I can help set up the phage side, they can do the rest. But I just started there, and so joining a new lab I ask, “what do we have, what do we not have?”
Ross:
—if you’re a lab, you have your fridges, your vendor, your postdocs and researchers, etc.
Jessica Sacher:
Yeah, and I’ve been in this phase of stepping back and asking, “is this going to proceed in the same way or differently? What is actually needed; what do we have; what do we do well?” It’s a much more academic environment, with undergrad students just milling around. You can't imagine checking your endotoxin count and seeing next to nothing, but eager and excited students are knocking over your media and asking, “Oh, can I do the hands-on part?”
Ross:
—Or storing things in your sample fridge. There's a tweet of yours I love, saying “Very important pro tip for your lab: buy your own fridge.”
1/ Biggest mindset shift:
— Jessica Sacher, PhD (@JessicaSacher) November 13, 2024
Expensive samples deserve their own private freezer.
Yes, really.
"But our lab already has a freezer!" isn't good enough when you're storing irreplaceable samples from a $2M grant 💸
Jessica Sacher:
(laughs) yeah! I want a lock on my fridge—and don’t get me wrong; I love the students, they’re so excited! But like they’ll just pick up a vial of phage and say, “This says T4. I think I can use it because my project’s about T4!” I’m going to have to start labeling things differently just to hide them. There’s a point where it’s not compatible with academia to have an operational mini pharmaceutical unit for phage therapy. We got to have that at Westmead because it was a research institute without students—we had one PhD student but no undergrads. I didn’t have to mentor them—which I really enjoy, but I had a lot of time back then!
We had the gene therapy center and so I could go down there, and their machine is validated and they have a quality manager. And now I have to develop the assay because we don't have the machine and the assay need’s troubleshooting and I have to be the one to say, “this is safe.”
So what aspect can we get good at that fits this environment? This lab has a lot going for it on the preclinical animal model and delivery side—getting the phage to the place—that's one of the factors on the table of failed trials. Knowing things like “can breathe it in if the target’s in the lungs, can put it on the burn wound if it’s on your skin, can do IV to get anywhere else”, but there’s some data coming through that the circulatory system gets rid of stuff, so there’s better ways if you can find them.
One of the things this lab is doing is hydrogels. They work with a group that’s engineering new slow release gels—they stick to the phages and then release them over the course of a week, so it’s as if you were dosing 10^9 phages daily.
Ross:
This is for injected, you said?
Jessica Sacher:
This is for topical but you can inject it under the skin, and it could be injected other ways, but not in the blood stream. locally, in a sense. But the important thing is that it lets you not treat as often for the same effect.
We might try to position ourselves as a center for phage deliver—we have the animal models all set up, which very few labs have, so maybe we do that instead of me being like “I’m making phages for each individual patient” and taking one step more zoomed out.
Ross:
So what’s the next bottleneck? What’s the next step trials fail at?
Jessica Sacher:
Yeah, its almost like I'm re-updating my OS—first 5 years it was about convincing doctors, then it was producing phages at all, and now that others can make the phages—I’ve blogged all my secrets online—but now its about getting them to the point of making them more predictable for a trial. We’re also writing grants for potential trials, trying to find a version of this story that resonates with whoever we tell it to that lets us make the next little steps forward.
But I’m also looking for the real bottleneck, and maybe it’s understanding clinical trial design. I’m not sure, but I am wondering, “why hasn't this happened yet, why don’t we have this?” It’s not just the phage field that’s doing stuff new, I’m very interested in soaking up all the other fields and how they’re doing things differently—especially CAR-T cell therapy, but also that’s probably the boring version of what’s on the cutting edge now.
Ross:
Yeah. What you hear about is the stuff that’s just now making it to you.
Well, this has been so much fun, and I’m excited to see what you’re doing next, where the field of phage therapy is going next, and how they (or you) pick apart these problems we think are keeping it from helping the people who need it.
Jessica Sacher:
Yes. Thank you, Ross; this was so much fun.


